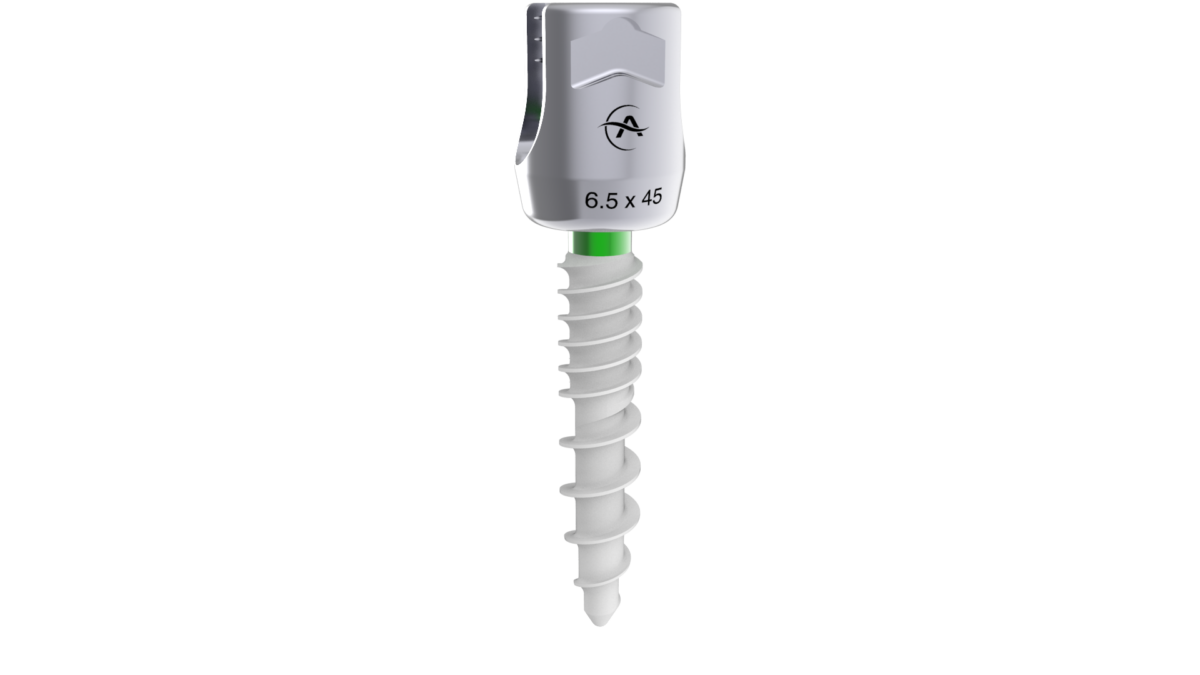
Altus Spine Announces FDA Clearance of Monaco HA Pedicle Screw System
Monaco HA Pedicle Screw System, consisting of a low-profile construct and insertion devices, features a new optimized surface intended for enhanced fixation. The Monaco HA screws have hydroxyapatite (HA) coating over the entire thread length, largely consisting of calcium and phosphorus, intended to enhance the fixation between the pedicle screw and the surrounding bone.
“To optimize fixation, our team is utilizing a hydroxyapatite (HA) coating over the screw thread – a game changer when it comes to thread purchase and pull-out strength.” said Michael Fitzgerald, President and CEO.
Featuring many of the same impressive qualities as Monaco Pedicle Screw System, Monaco HA includes a friction fit screw head so it remains in the appropriate position for rod implantation and a small outer diameter for optimal bone graft placement.
The FDA’s action means that the HA Pedicle Screw System is now indicated to provide immobilization and stabilization of spinal segments in skeletally mature patients as an adjunct to fusion in the treatment of acute and chronic instabilities or deformities of the thoracic, lumbar, and sacral spine. The Altus Spine HA Pedicle Screw System is intended for noncervical pedicle fixation for the following indications: degenerative disc disease (defined as back pain of discogenic origins with degeneration of the disc confirmed by history and radiographic studies); spondylolisthesis; trauma (i.e., fracture or dislocation); spinal stenosis; curvatures (i.e., scoliosis, kyphosis, and/or lordosis); tumor; pseudoarthrosis; and failed previous fusion in skeletally mature patients.
“This FDA clearance is a huge step in expanding our dynamic and innovative solutions to provide immobilization and stabilization in the thoracic, lumbar, and sacral spine,” said Fitzgerald, “Our goal is to continue advancing and creating top of the line, world-class products with the latest technologies to provide better outcomes for our patients.”
Altus Spine
Based in West Chester, PA, Altus Spine is dedicated to creating the next generation of medical devices. Altus strives to improve patient care by designing and manufacturing products to meet the highest standards in an ever changing and evolving field. Implemented and used by over 100 hospitals across the United States, Altus is among the fastest growing spinal implant companies in the world. For additional information, or to inquire about distribution opportunities, please contact us at info@altus-spine.com.
Forward Looking Statements
All statements made in the above press release, with the exception of historical fact, may be forward-looking statements that include risk, uncertainty, and assumptions. These factors could cause Altus Spine’s results to differ from those predicted if they do not occur as expected. These uncertain factors include, but are not limited to: acceptance and clearance of the company’s surgical products and procedures, development of new products and procedures, innovations and alterations to existing products and procedures, clinical and statistical verification of the success using Altus Spine’s products, company’s ability to maintain and monitor inventory as it releases new products, its ability to hire and retain personnel, and any other risks stated in prior or subsequent news releases. All risks and potential complications can be found in our most recent 510(k) report from the Food and Drug Administration (FDA). Given the constantly changing market, readers are encouraged to not place undue reliance on forward-looking statements. Altus Spine assumes no obligation to update forward-looking statements as these changes occur, or as events and circumstances are altered, after the date they are posted.