About
Mission
Altus Spine is dedicated to improving patient care through innovative engineering, surgeon collaboration, and world class manufacturing. We are driven to advance clinical outcomes by delivering advanced procedural solutions.
Accelerated Delivery
The performance capability of the Altus Spine manufacturing plant is driven by our customer focus. Our multi-disciplinary expert engineers utilize the plant’s proficient power to rapidly deliver to customer requests and uncompromising market demands.
Manufacturing Excellence
Every employee at Altus is focused on our three core values: quality, speed and safety. We house the most modern equipment and state of the art technology to manufacture our premium products.
Quality Commitment
We are committed to providing the medical device community with superior quality spine products that exceed our customers expectations through rapid innovation and compliance to industry standards.
We achieve this through targeted customer focus, continuous improvement, and a highly effective production management system delivering to ISO regulatory requirements.
News
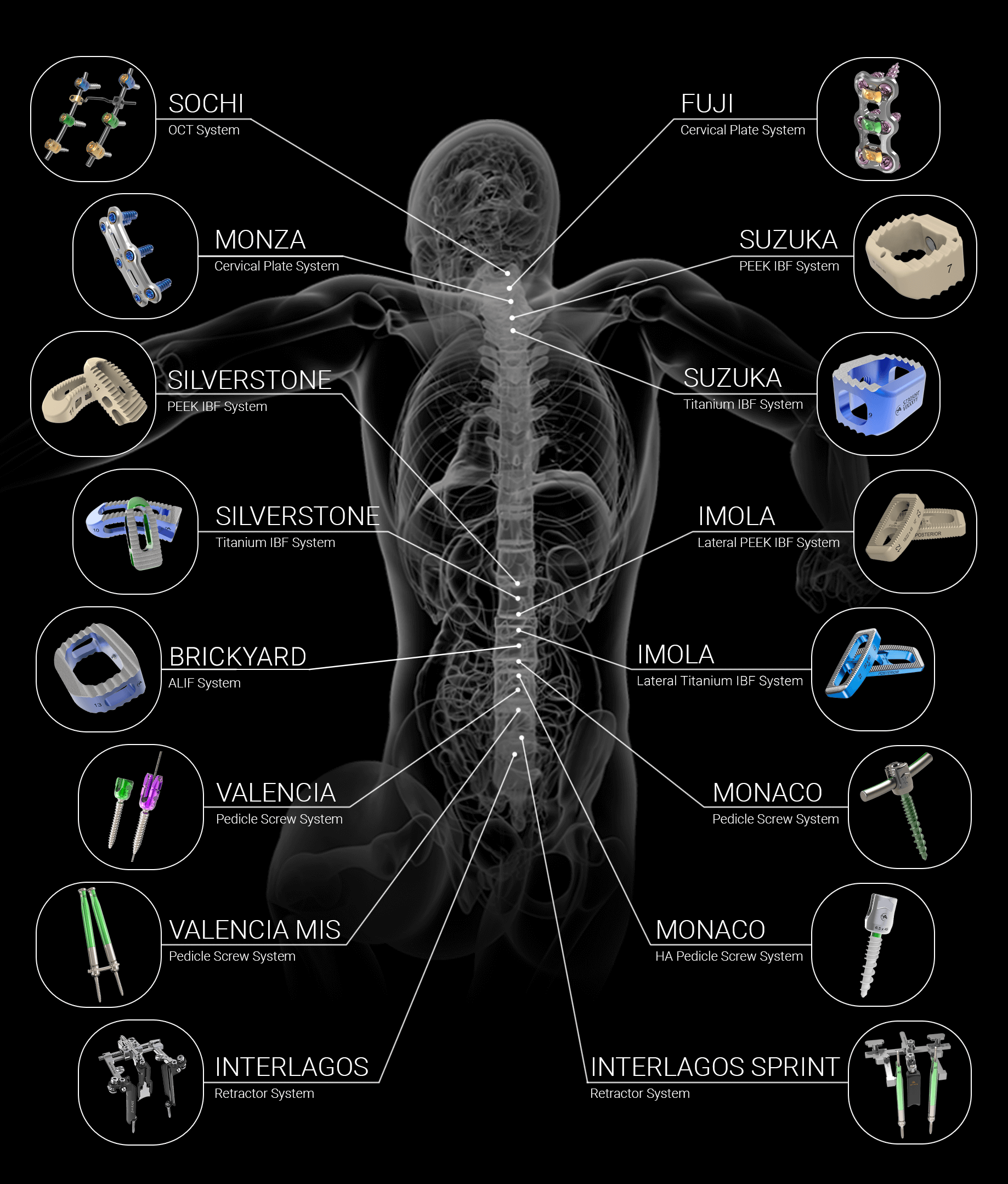
Altus Spine Announces FDA Clearance of Sochi OCT System
West Chester, PA – Tuesday, July 8th, 2022 – Altus Spine, a leader in the development and innovation of medical devices used in spinal correction surgery, announces FDA 510(K) clearance of Sochi OCT System. The Altus Spine Sochi OCT System provides a seamless transition from…
Altus Spine heads to Prague, Czech Republic, as Platinum Sponsors of the Society of University Neurosurgeons Annual Meeting!
West Chester, PA – Monday, June 6th, 2022 – Altus Spine, a leader in the development and innovation of medical devices used in spinal correction surgery, announces Platinum Level Sponsorship of the Society of University Neurosurgeons Annual Meeting, being held June 29th-July 3rd, 2022. Located…
Altus Spine Announces FDA Clearance of Monaco HA Pedicle Screw System
West Chester, PA – May 31, 2022 – Altus Spine, a leader in the development and innovation of medical devices used in spinal correction surgery, announces FDA 510(K) clearance of Monaco HA Pedicle Screw System. Monaco HA Pedicle Screw System, consisting of a low-profile construct…
Featured Jobs
We're Hiring!
Surgical Support Spine Specialist
Altus-Spine is seeking a Surgical Support Spine Specialist who will understand the clinical expectations of our healthcare professionals and proactively anticipate their needs. In this role, you will complete a training program to learn the anatomy of the spine, procedural treatments, spinal repair products and effective... Learn More!
Design Engineer, Spine
The Design Engineer - Spine serves as the senior technical specialist and project leader for spinal surgery system development teams. The position is “hands on” with the primary goal being to establish product and system requirements, create project development plans, and to design and manage the... Learn More!
Inventory Analyst
Altus Spine is pursuing the best and the brightest in our industry. The Inventory Analyst - Medical Device ensures that all surgical operations are fully equipped with necessary Altus equipment to accommodate the scope of the surgical case(s) including surgeon preferences when possible. He/she will participate in... Learn More!
Senior Design Engineer
Altus Spine is pursuing the best and the brightest in our industry. We have the ideal landing spot for the experience engineer waiting for the right opportunity to lead their own team and create a substantial impact in our business. If we are talking about you, we encourage you to submit an application for this position... Learn More!